nQuery is the world’s most trusted clinical trial design platform
The complete trial design platform to make clinical trials faster, less costly & more successful
Receive Regulatory Approval
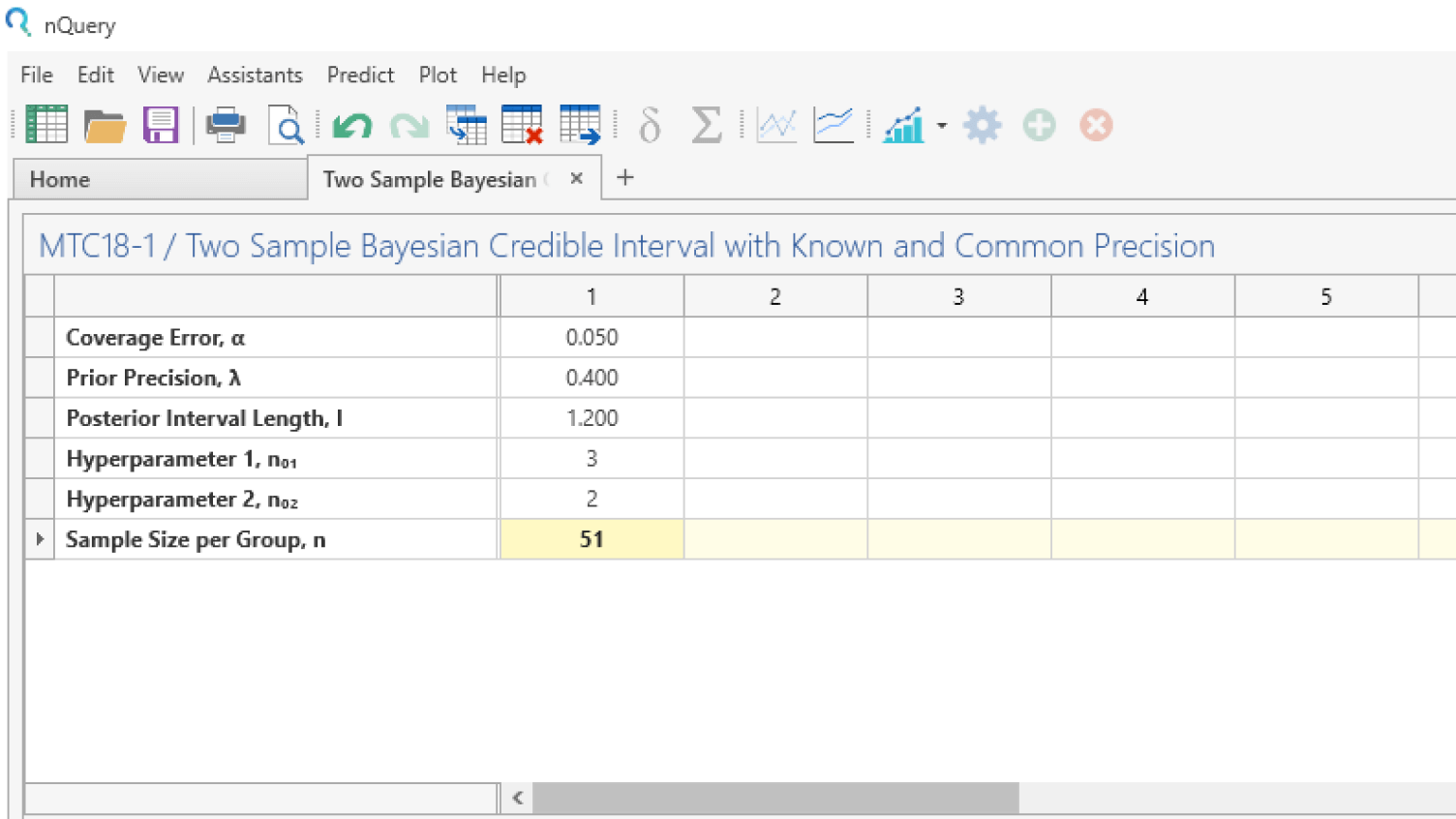
Powerful Sample Size options for FDA/EMA submission
- Align your sample size with scientific & budgetary requirements
- 1000+ validated sample size and power calculation procedures
- Sample size statement generator

Adaptive Trials
Adjust your clinical trial based on study data
- Design efficient, informative and ethical adaptive clinical trials
- Includes interim analyses, sample size re-estimation and MAMS
- Optimize financial resources
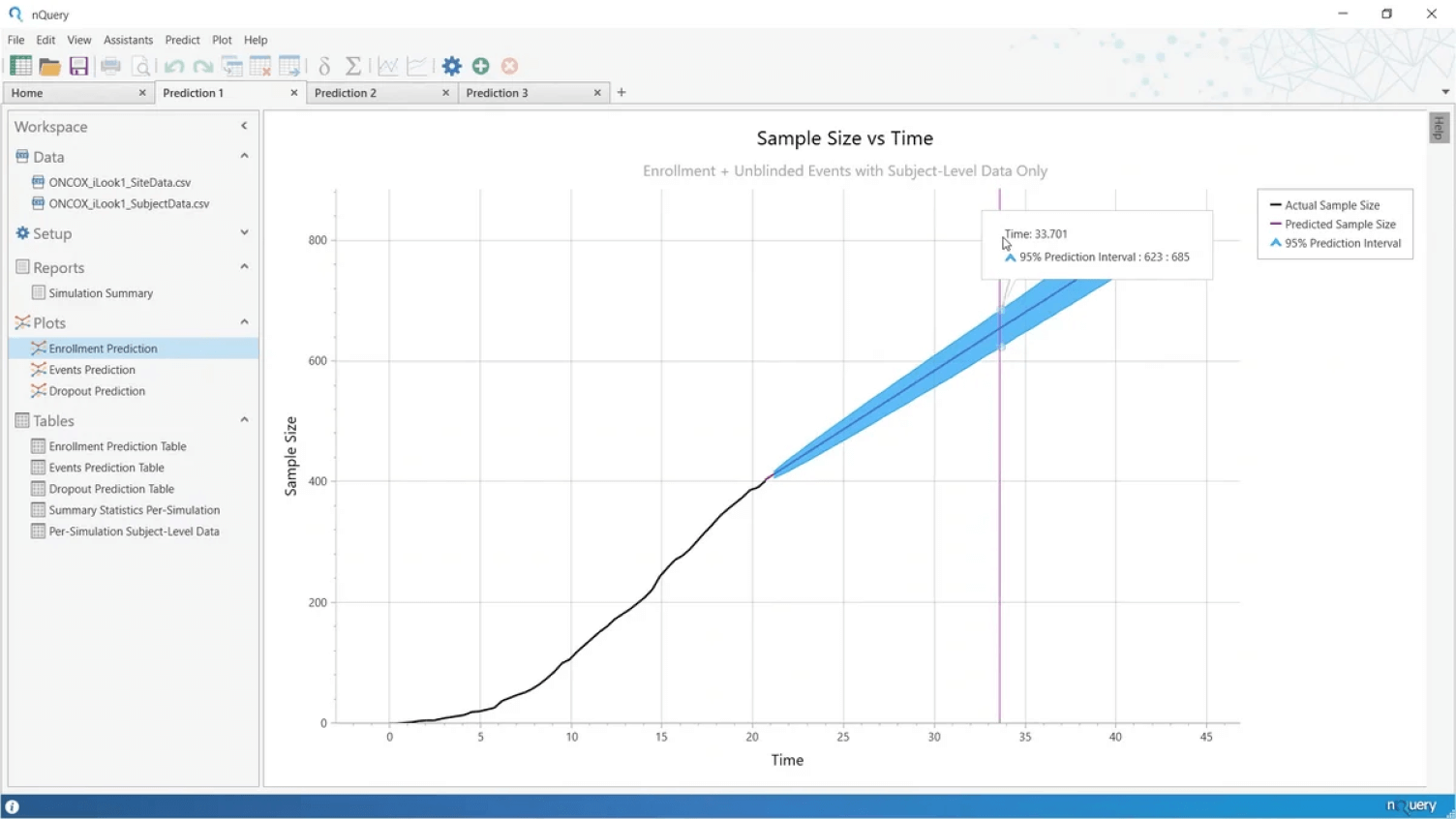
Key Event Prediction
Accurately predict your key trial milestones
- Identify roadblocks and take action to keep your trial on schedule
- Use your trial data to project when key milestones will be reached
- Projections for both blinded and unblinded survival data
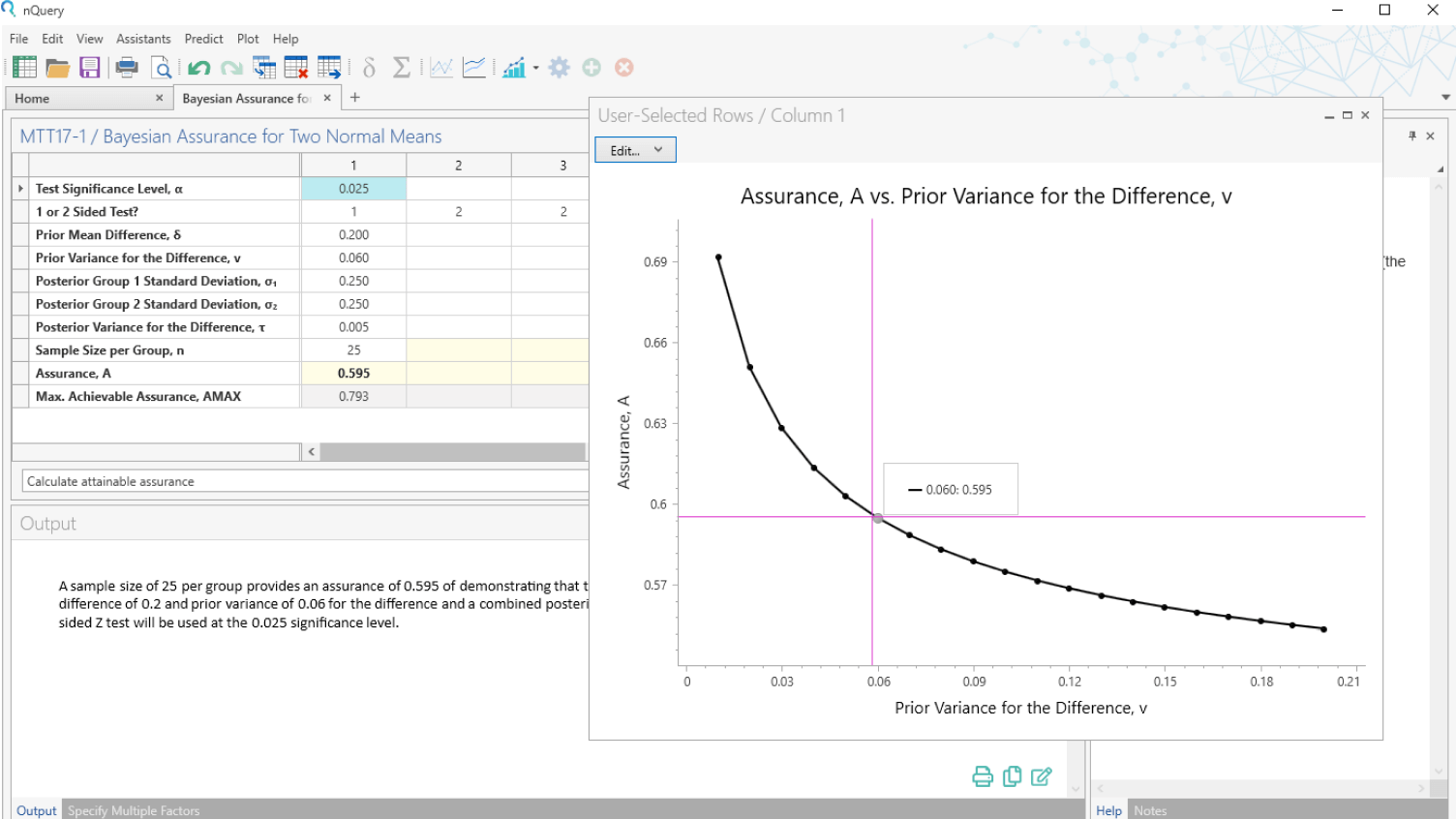
Bayesian Statistics
Integrate prior information, real-world data & expert opinions
- Bayesian assurance - the true probability of success
- Justify complex Bayesian methods to non-statisticians
- Identify study design threats & opportunities
Recommended Resources
Get started with nQuery today
Start for free and upgrade as your team grows








