What's New in the nQuery Base Module
The Winter 2019 release extends the number of tables on offer for classical trial designs. 13 new tables have been added.
In this release, the main areas are:
- Count/Incidence Rate Models
- Post-Marketing Surveillance
- Non-Inferiority Log Rank
Count/Incidence Rate Models
Count and incidence rates are a common type of data where the endpoint of interest is the number of events that occur in a given unit of time or repeated counts. Examples in clinical trials include the exacerbation incidence rate in a given year for respiratory diseases such as COPD or the count of the number of lesions found in MRI scans for conditions such as multiple sclerosis. Count models such as Poisson or Negative Binomial allow the full usage of all events found compared to binomial or survival modelling approaches.
4 tables will be added:
- Poisson Cross-over Designs (2x2, Stepped Wedge CRT)
- Andersen-Gill Model
- Negative Binomial Model
Poisson Cross-over Designs (2x2, Stepped Wedge CRT)
The Poisson distribution is popular for modelling the number of times an event occurs in an interval of time or space. The Poisson distribution is characterized by a single parameter which is the mean number of occurrences during the specified interval.
In a crossover design each subject receives all treatments at least once with the objective of measuring study differences among the treatments. “Crossover” comes from the most common two treatments case which is of interest here. Crossover designs are popular due to an expected increase in precision, since each subject effectively acts as their own control, and the concomitant reduction in study size but do have additional statistical and practical complications such as carry-over effects.
The stepped-wedge cluster randomized design is a cross-over design in which each cluster, rather than subject, receives both the treatment and control. Cluster randomized trials (CRT) are trials where the assigned treatment is applied to all subjects selected in a cluster rather than random assignment within each cluster. For a complete stepped-wedge design, all clusters are initially assigned to the control group and a fixed number of clusters switch to the treatment group for the rest of the study with different subjects measured within each cluster and no subject is measured more than once. Stepped-wedge designs are useful in cases where it is difficult to apply a particular treatment to half of the clusters at the same time.
Cross-over designs for count/rates data is provided for the Poisson model in this release for the inequality, non-inferiority and equivalence cases. The stepped-wedge CRT for Poisson count/rate data is provided in this release for the inequality case.
4 tables will be added:
- Two Poisson Cross-over Non-inferiority
- Two Poisson Cross-over Equivalence
- Two Poisson Cross-over Inequality
- Stepped Wedge CRT Poisson Inequality
Anderson Gill Model
The Andersen-Gill (AG) generalizes the Cox model from the single event (survival) to multiple event (counts/rates) context, with the rate characterized in terms of the increments of time between events. As per the semi-parametric Cox model, it assumes a common unknown baseline hazard function for events. This allows the AG model greater flexibility versus the Poisson and Negative Binomial models, which assume a constant event rate, while providing comparable results when the event rate is constant.
Sample size for comparing the rates/counts in two independent groups using the Andersen-Gill model assuming a Weibull hazard rate is provided in this release for the inequality, non-inferiority and equivalence cases.
3 tables will be added:
- Non-inferiority Test for Ratio of Two Incidence Rates using Anderson-Gill Model
- Equivalence Test for Ratio of Two Incidence Rates using Anderson-Gill Model
- Inequality Test for Ratio of Two Incidence Rates using Anderson-Gill Model
Inequality for Negative Binomial Unequal Follow-Up
The Negative Binomial model is a generalization of Poisson regression which loosens the restrictive assumption that the variance is equal to the mean. Loosening this restriction allows the flexibility to deal with the common occurrence of overdispersed count data which would cause lower power and accuracy for the Poisson model. Most negative binomial regression models (e.g. NB2) are based on the Poisson-gamma mixture distribution.
Sample size for comparing the rates/counts in two independent groups using the Negative Binomial is provided in this release for the inequality cases allowing for unequal follow-up times, dropout and dispersion parameters.
1 table will be added:
- Inequality Test for Ratio of Two Incidence Rates using Negative Binomial Model
Post Marketing Surveillance
Postmarketing surveillance (PMS) is the practice of monitoring the safety of a pharmaceutical drug or medical device after it has been released on the market and is an important part of the science of pharmacovigilance. Postmarketing surveillance can further refine, or confirm or deny the safety of a drug or device, after it is used in the general population who will have a wider variety of medical conditions than seen in a confirmatory clinical trial.
Four potential PMS designs are provided in this release with three cohort designs, with different assumptions about the adverse reaction rate, and the case-control design.
4 tables will be added:
- Post-Marketing Surveillance with No Adverse Reaction Rate
- Post-Marketing Surveillance with Known Adverse Reaction Rate
- Post-Marketing Surveillance with Unknown Adverse Reaction Rate
- Post-Marketing Surveillance for Matched Case-Control Study
Non-Inferiority Log rank Test
The log-rank test is one of the most common statistical tests used for the analysis of survival data. It’s flexibility and interpretability provide useful insights into the comparable hazard rates in survival clinical trials.
Non-inferiority tests are used to statistically evaluate if a proposed treatment is not worse than a pre-existing standard treatment by testing if the result is statistically better than a specified non-inferiority margin. This is a very common objective in areas such as generics and medical devices.
Sample size for the non-inferiority log-rank test comparing two independent survival curves is provided here with flexibility to allow the alternative hypothesis hazard ratio to differ from 1 (i.e. equal survival rates per group).
1 table will be added:
- Non-inferiority Log-Rank Test (non-1 HR default)
New Home Screen & User Interface Updates
The Winter 2019 update sees numerous small but great user interface improvements to nQuery. The first one you will notice is the great new home screen. Here you will have quick access to a host of common tasks in addition to easily seeing what nQuery Packages you have.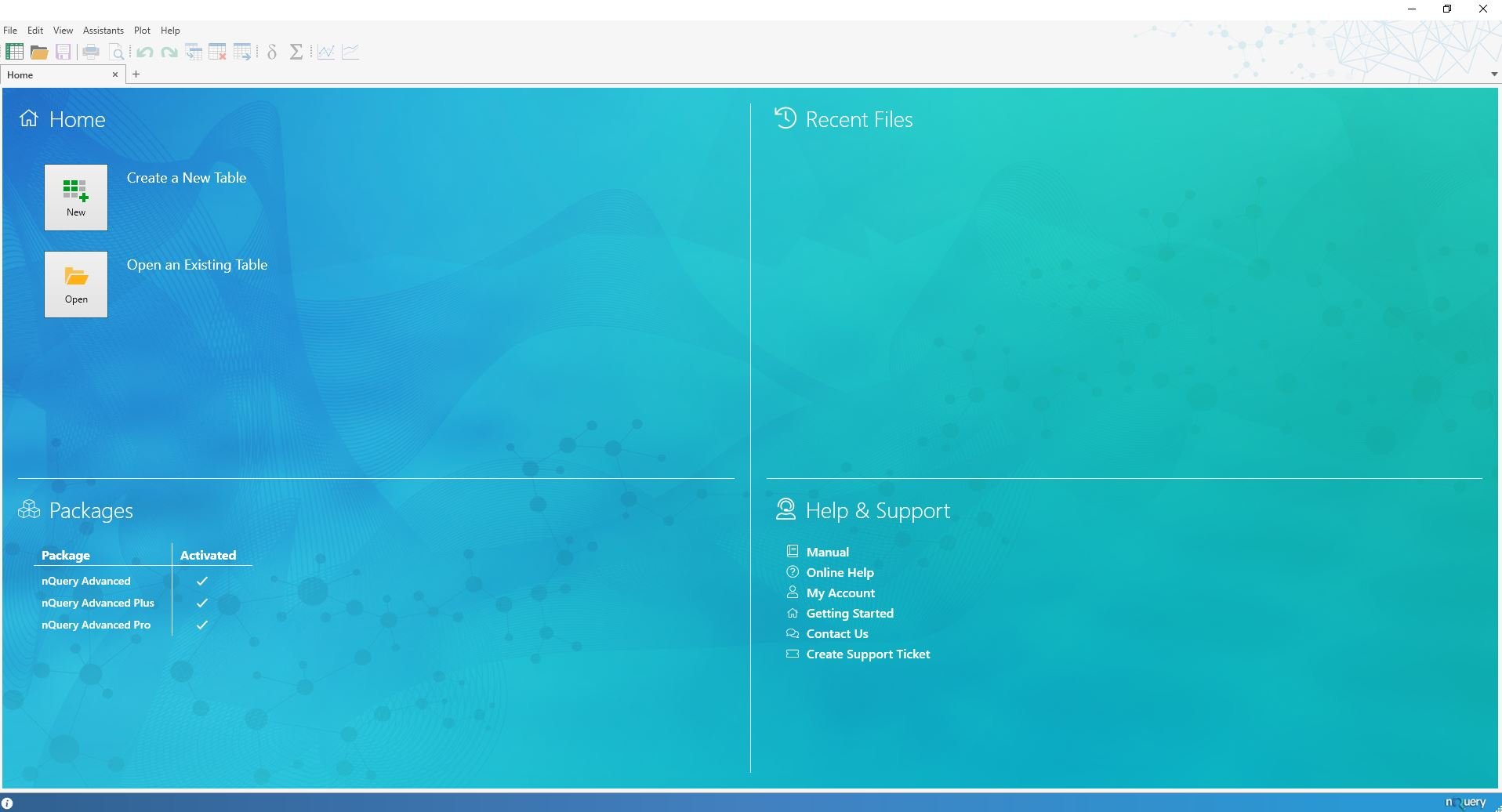
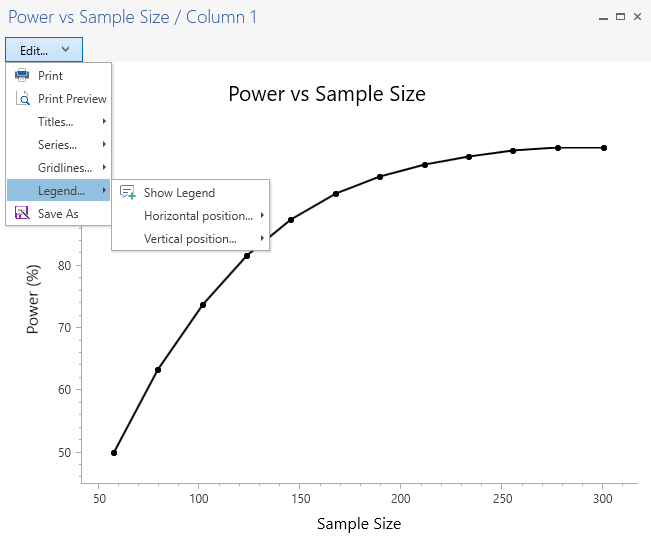
Updated Graphing Tool
The graphing tool in nQuery has also been updated for greater flexibility for the user. The default color scheme is now black and white with many options to customize graphs. The new powerful graphing tool makes graphs exported from nQuery perfect for regulatory and academic submissions.