Customers
Optimizing clinical trial design for innovative organizations
Case Studies
We help our customers optimize their clinical trial designs to save costs and reduce risk
Learn more about their successes.
Adaptive Trial Designs in nQuery - Effective and Efficient Development
Managing cost, risk, and competition in drug development
Read More
Advancing patient care for Japan's most common cancer
Calculating the appropriate sample size for a first of its kind stage-II colon cancer trial
Read More
What our users say

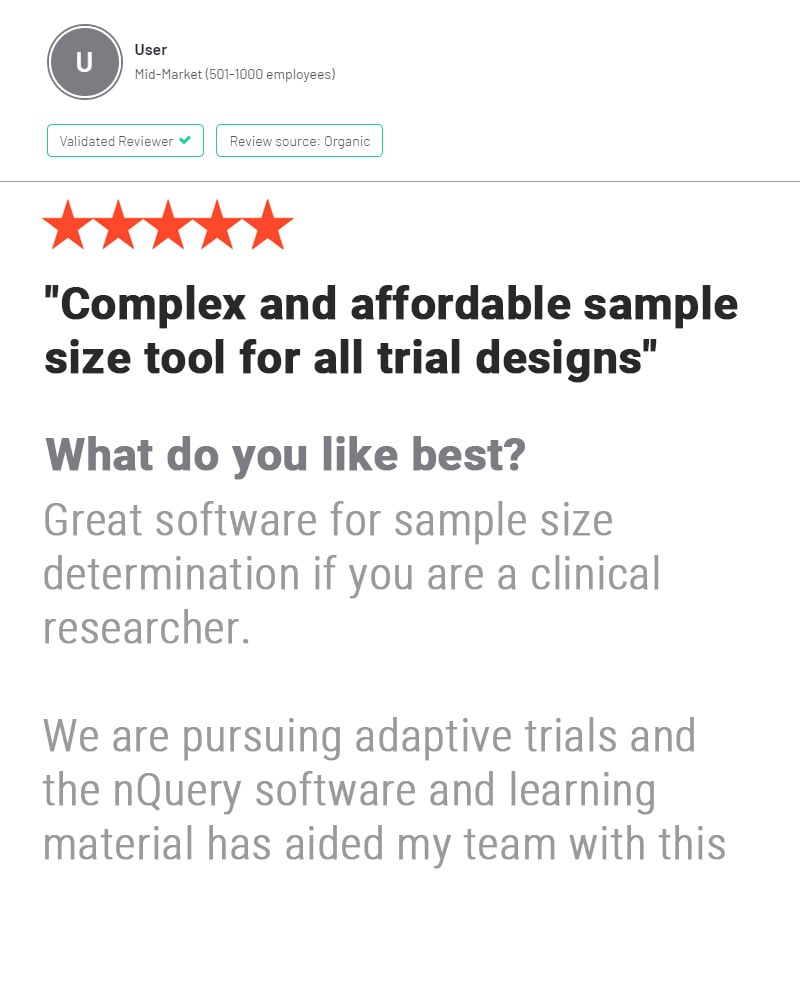
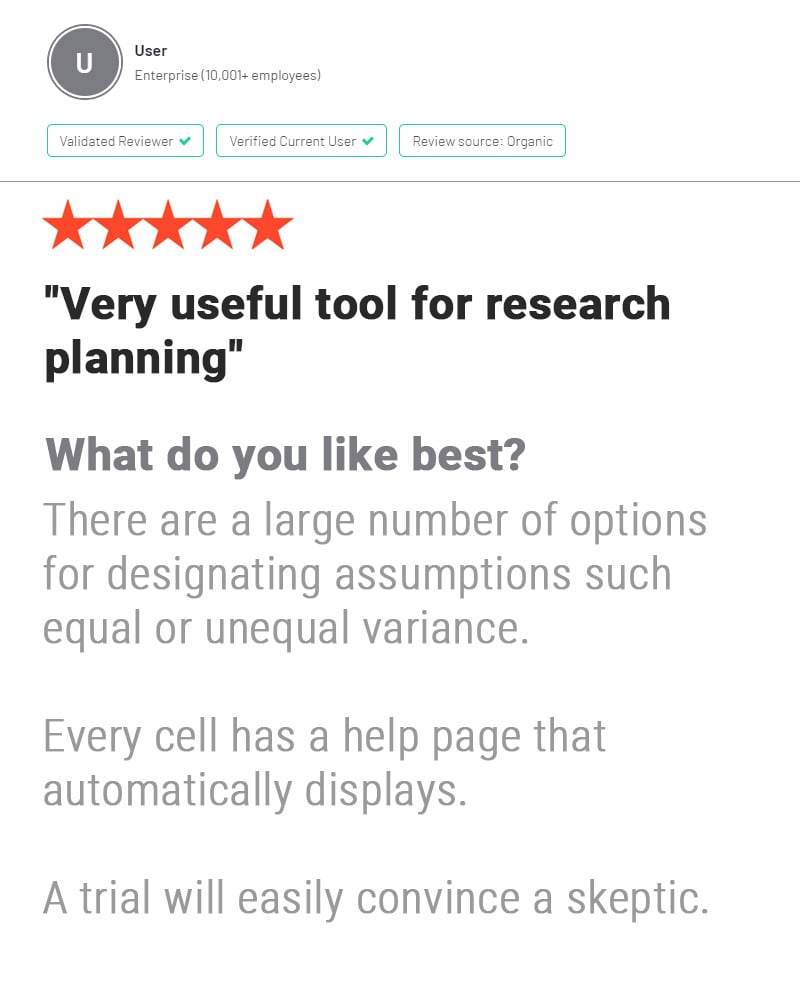
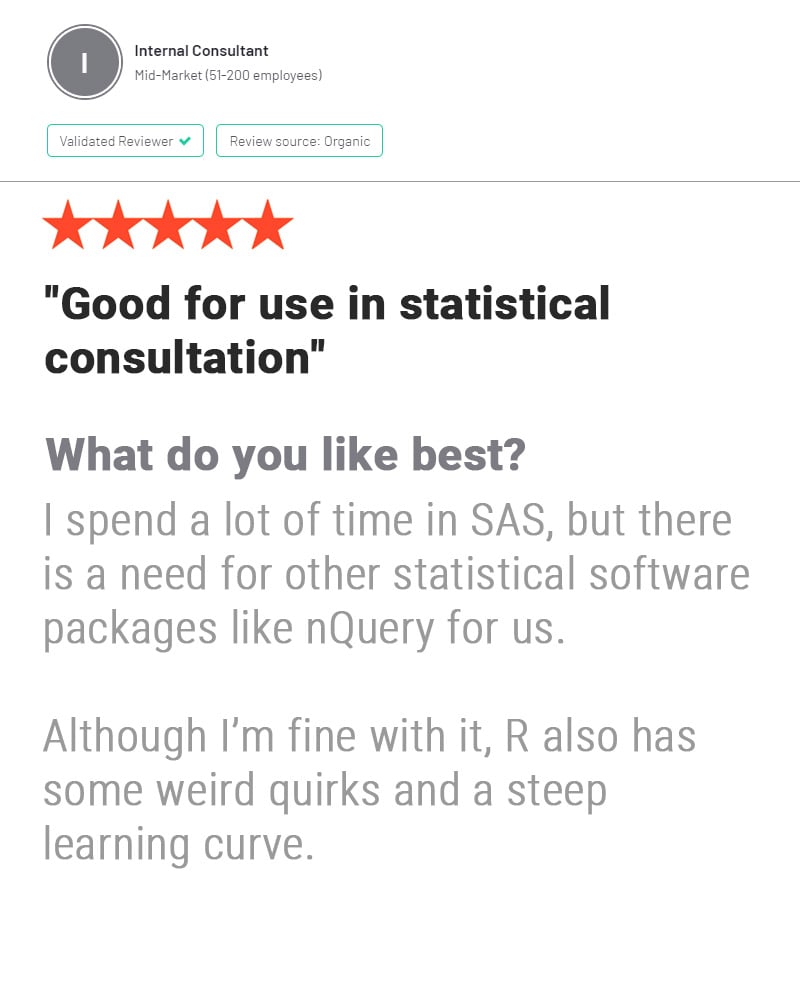
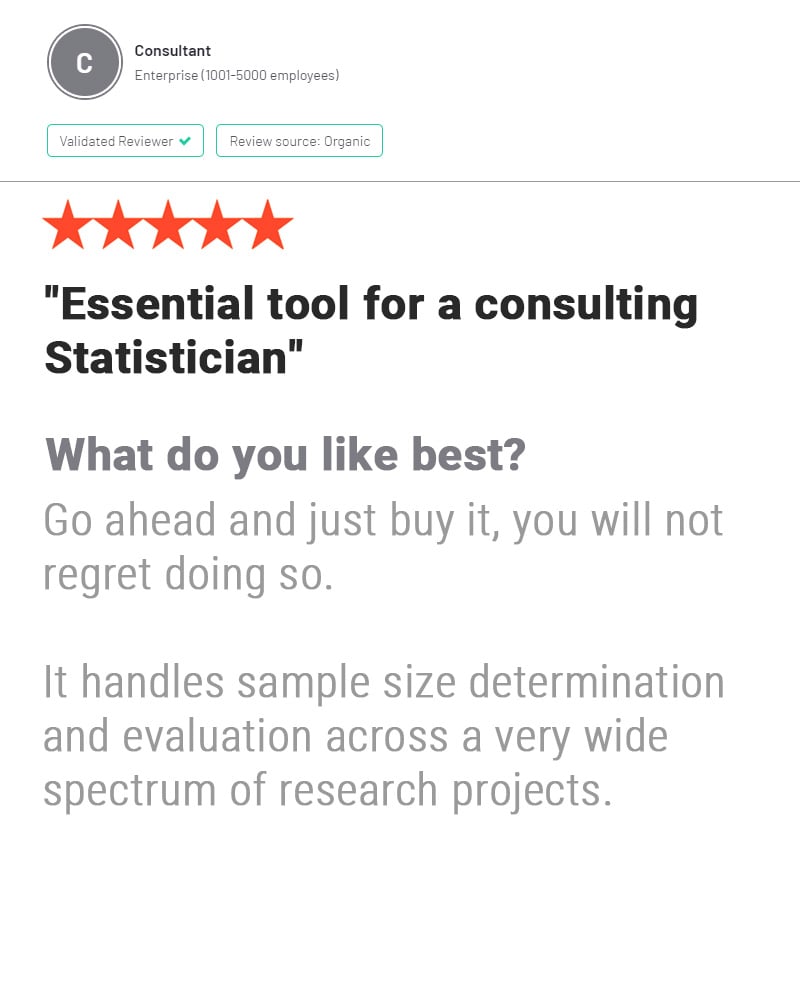
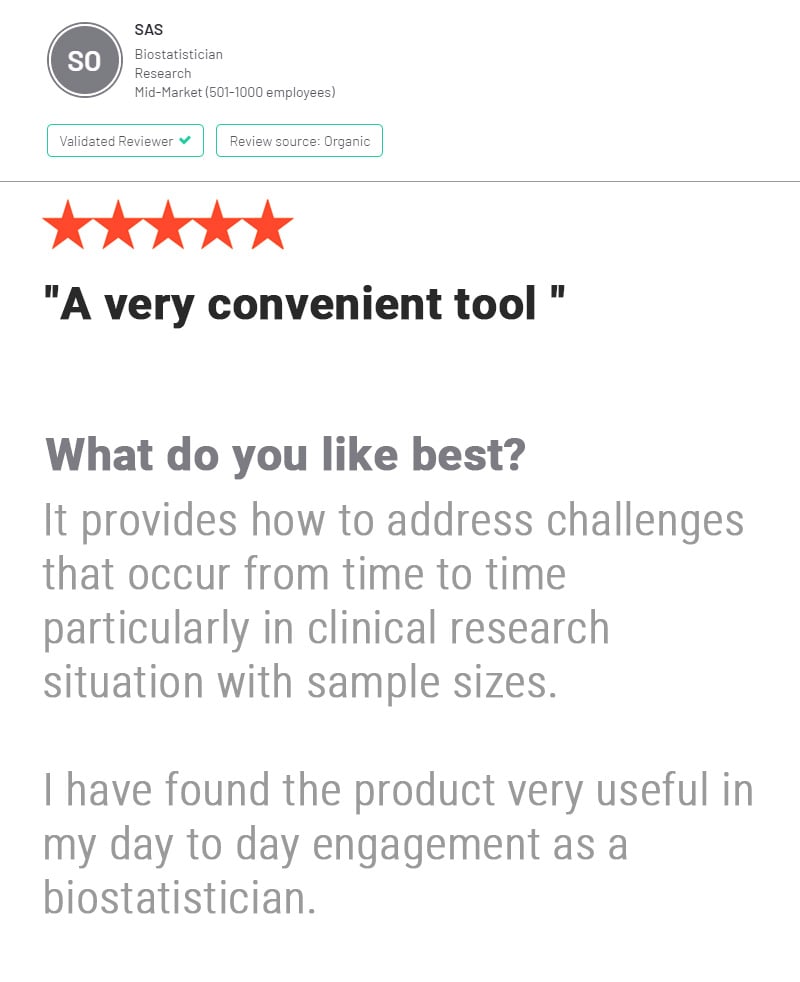
nQuery is used by biostatisticians and clinical researchers in clinical trial design to save costs and reduce risk
Commercial Clients
Pharmaceutical, Biotech & Clinical Research Organizations

Academic Clients
Used in Universities for Teaching and Research

Government Clients
Government Health Departments Worldwide

See how nQuery is used
Real clinical trials demonstrated in nQuery
- Improve your sample size calculations with nQuery clinical trial design templates and data.
Get started with nQuery today
Start for free and upgrade as your team grows