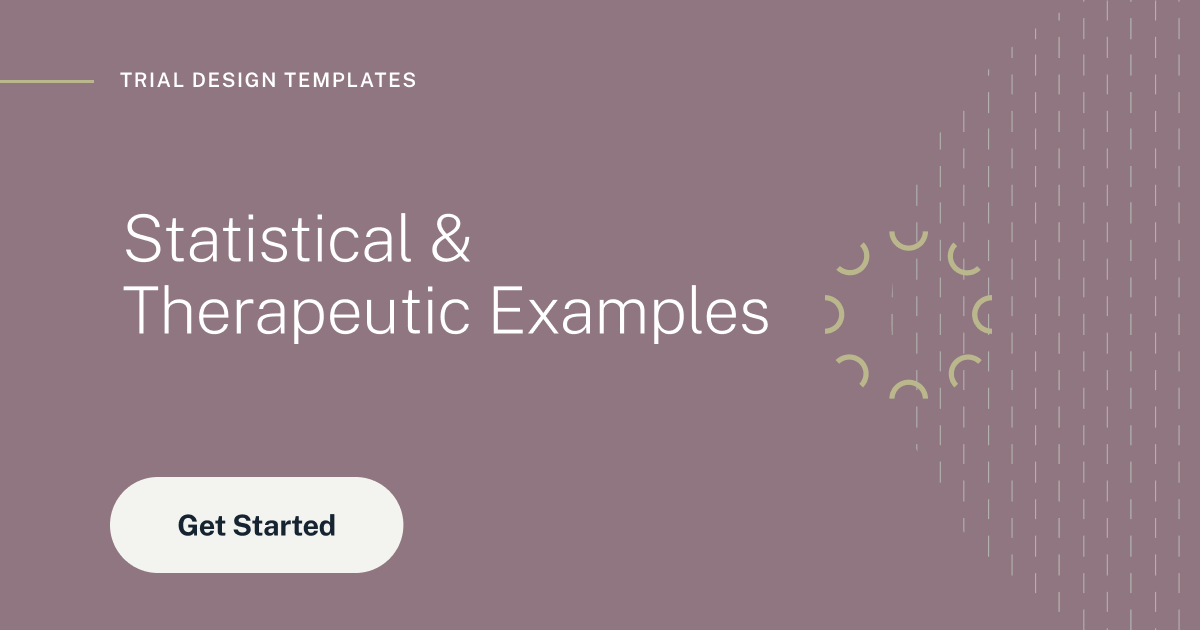
The Clinical Trial Design Platform for Sample Size, Bayesian & Adaptive Trials
nQuery is used by biostatisticians and clinical researchers to save costs and reduce risk, by helping them optimize clinical trial designs
Receive Regulatory Approval
Powerful Sample Size options for FDA/EMA submission
- Align your sample size with scientific & budgetary requirements
- 1000+ validated sample size and power calculation procedures
- Sample size statement generator

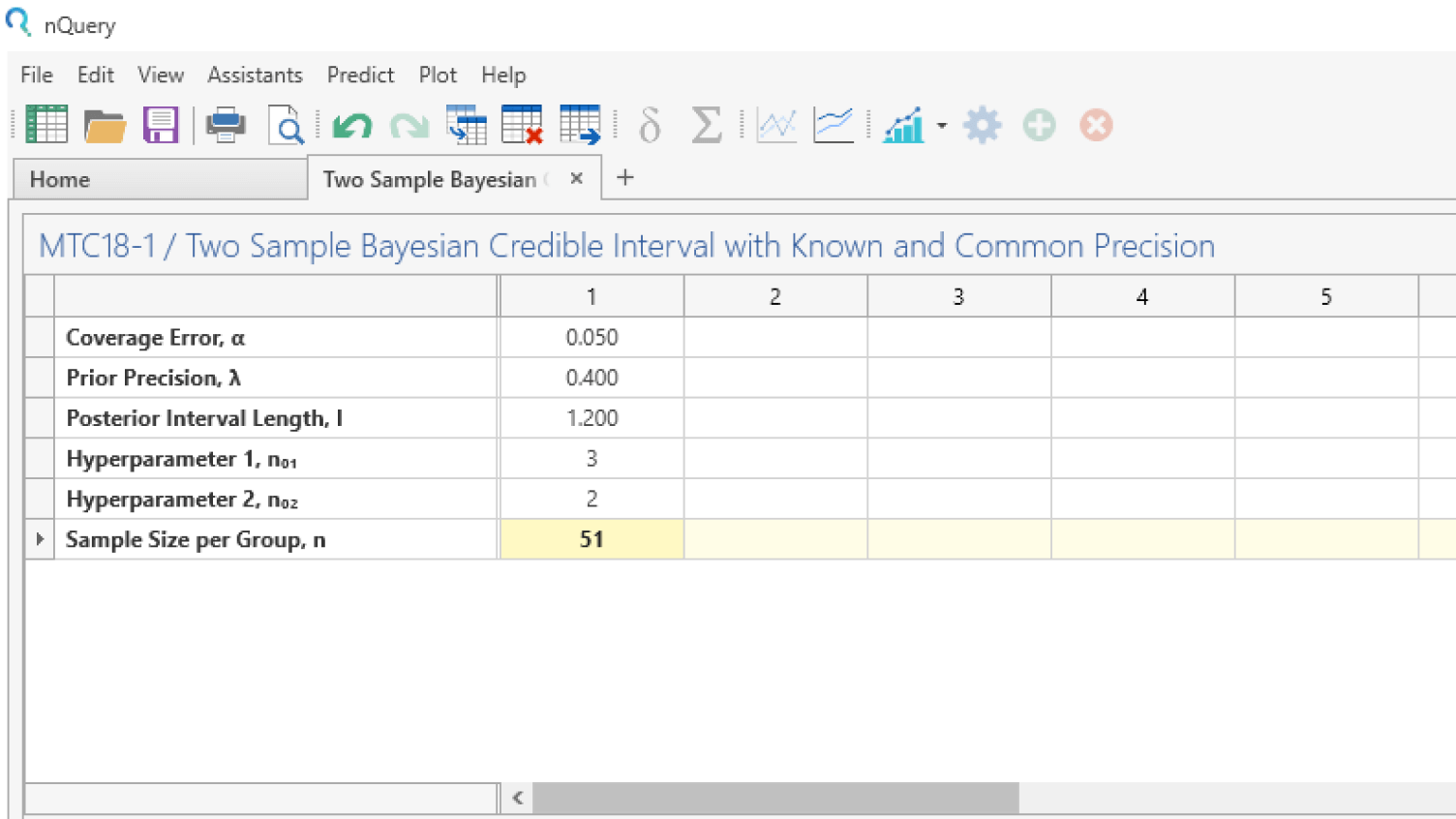
Adaptive Trials
Adjust your clinical trial based on study data
- Design efficient, informative and ethical adaptive clinical trials
- Includes interim analyses, sample size re-estimation and MAMS
- Optimize financial resources
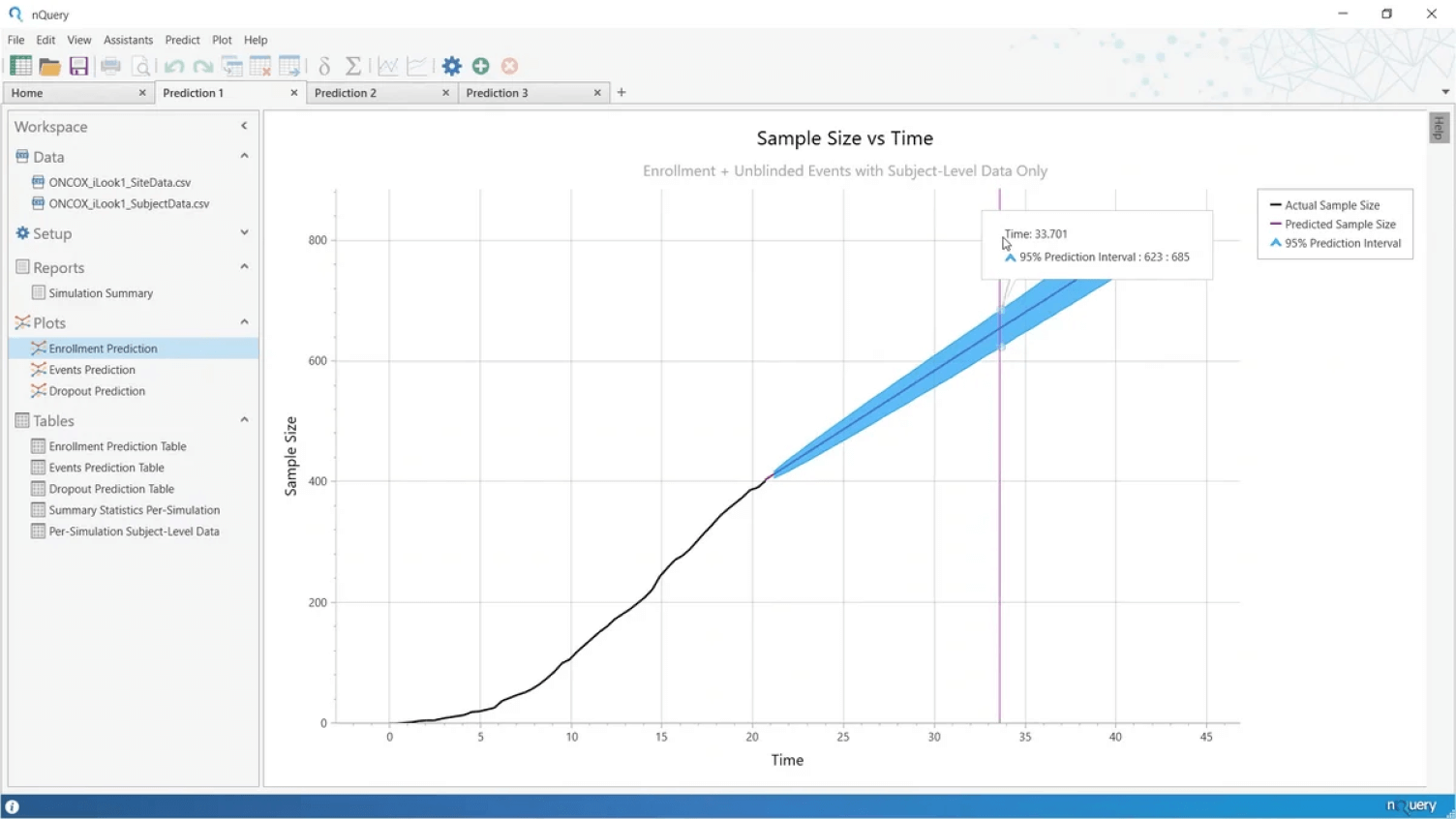
Key Event Prediction
Accurately predict your key trial milestones
- Identify roadblocks and take action to keep your trial on schedule
- Use your trial data to project when key milestones will be reached
- Projections for both blinded and unblinded survival data
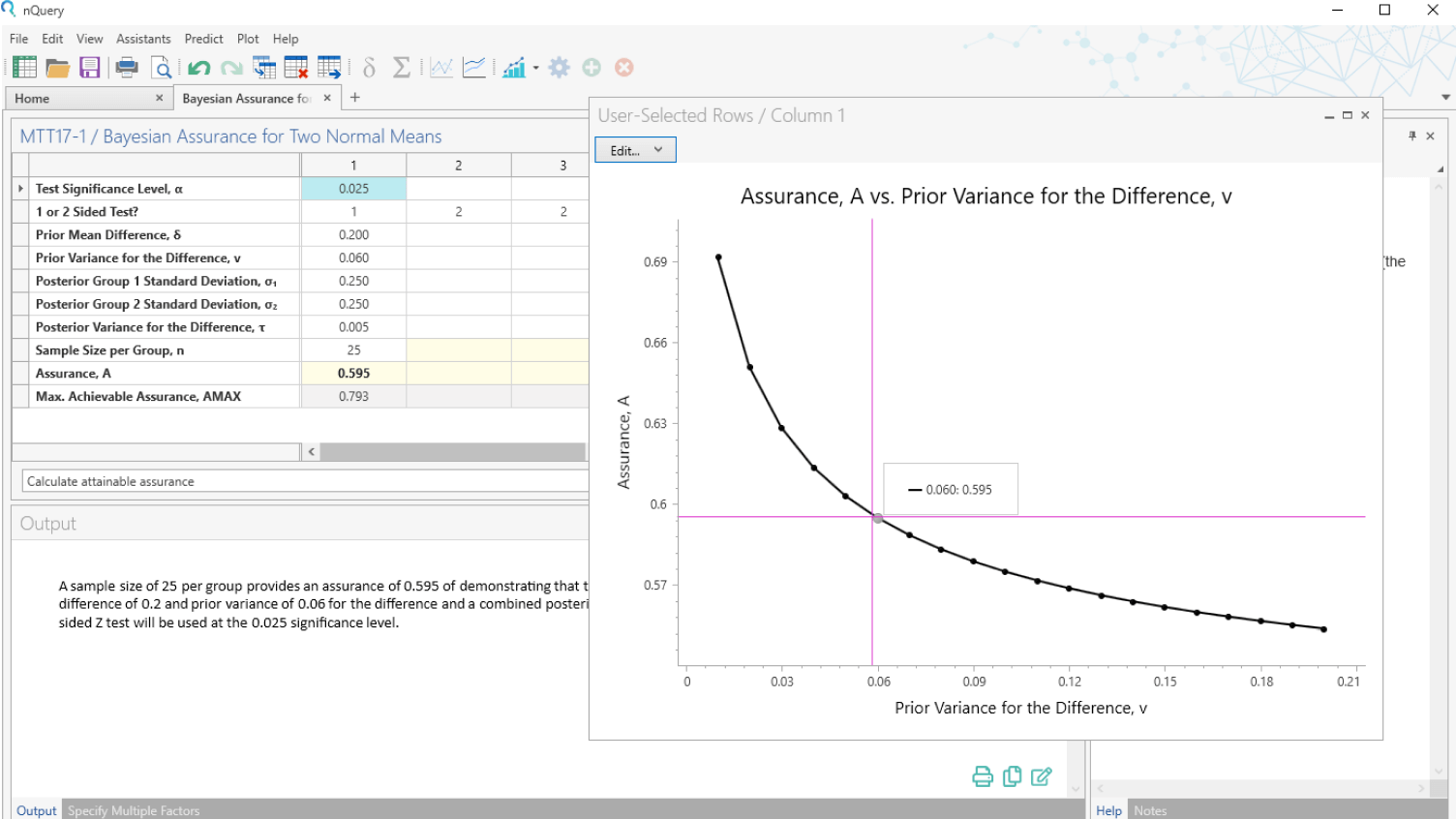
Bayesian Statistics
Integrate prior information, real-world data & expert opinions
- Bayesian assurance - the true probability of success
- Justify complex Bayesian methods to non-statisticians
- Identify study design threats & opportunities
Recommended Resources
Get started with nQuery today
Start for free. Upgrade as your team grows